Wir sorgen dafür, dass Ihre
Produkte in Asien perfekt produziert und
genau nach Ihren Vorgaben bei Ihnen ankommen.
Mehr InfoWir haben das Wissen über die Chemie der Produkte.
Wir wissen was getestet werden muss -
denn nicht jeder Test ist sinnvoll und kostet nur Geld...
Mehr InformationenWir kennen beide Seiten.
Die asiatischen Hersteller und die Ansprüche
unserer europäischen Kunden.
Mehr InformationenWir kümmern uns für Sie um eine einwandfreie
und rechtlich sichere Dokumentation
Ihrer Produktion in Asien.
Mehr InfoSie produzieren bereits oder planen, in Asien
zu produzieren? Wir unterstützen Sie dabei.
Die LGT Logistic and Goods Testing Institute GmbH ist ein Anbieter maßgeschneiderter Dienstleistungen zur Qualitätssicherung von technisch anspruchsvollen Produkten aus Asien. Effizient, wirtschaftlich attraktiv und dem hohen deutschen Qualitätsverständnis entsprechend schließen wir die Lücke zwischen den Erwartungen des Marktes und den Fähigkeiten der Lieferanten.
Dabei agieren wir überwiegend direkt beim Hersteller vor Ort und befreien unsere Kunden von allen zeit- und kostenintensiven Arbeiten, die mit der Beschaffung in Asien verbunden sind.
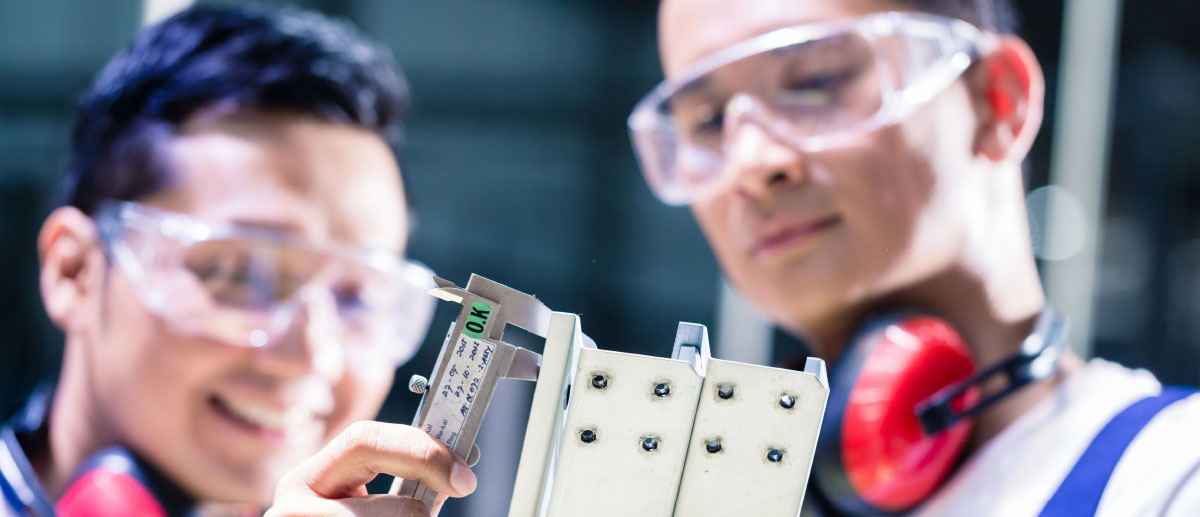
Produkt-Inspektion
Inspektion Ihrer Produkte während des Herstellungsprozesses und kurz vor Versand.
- Mehr Info
Produkt-Prüfung
Test Ihrer Produkte auf Normerfüllung, Chemische Unbedenklichkeit (REACH) und Produktzuverlässigkeit.
- Mehr Info
Produzenten-Entwicklung
Entwicklung der verfügbaren Hersteller zu verlässlichen Sourcing-Partnern.
- Mehr Info
Dokumentation
Technische Dokumentation der vom Gesetzgeber geforderten Informationen.
- Mehr Info
Full Service für Ihre Produktion in Asien.
Wir sind für Sie vor Ort und an Ihrer Stelle.
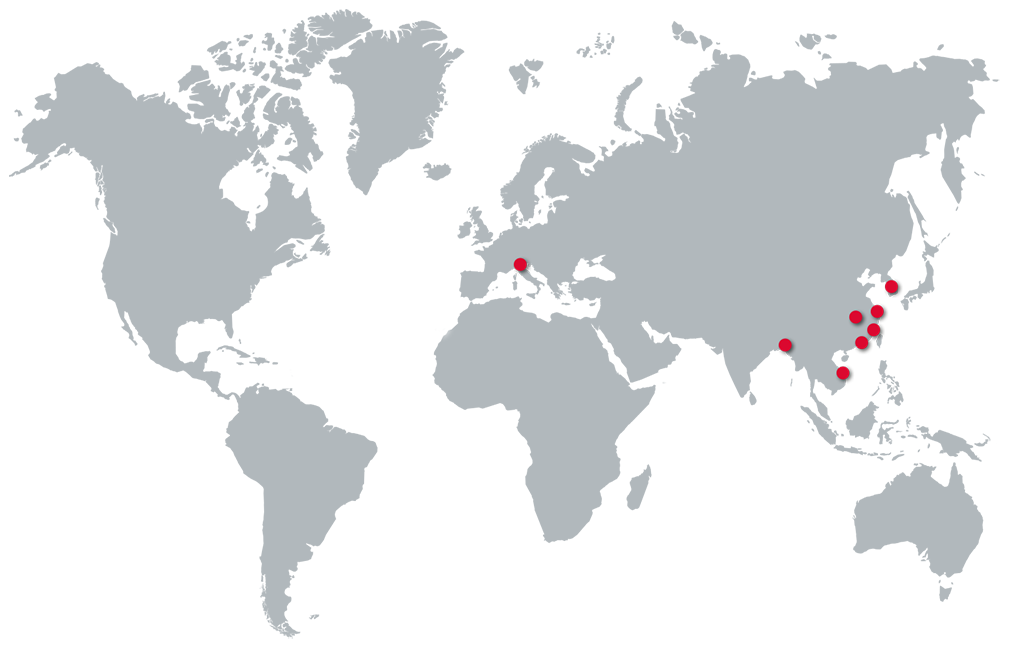
Unsere Teams sind für Ihre Produktionen in den wichtigsten Ländern und Regionen wie z.B. Wenzhou, Shenzen, Xiamen, Yingtan, Korea, Bangladesh, Vietnam sowie in Italien verfügbar und kümmern sich um die Produktionsüberwachung und Einhaltung Ihrer Qualitätsvorgaben.
Unsere Service- und Beratungsleistungen für Ihre erfolgreiche Produktion in Asien
Vertrauen in Ihre asiatischen Produzenten ist gut.
Wir wissen aus Erfahrung - Kontrolle ist besser.
Produzenten in Asien gibt es viele und richtig günstige.
Wir kennen oder finden den genau Passenden.
EU-Normen sind kompliziert.
Wir kennen uns damit aus.
Der Gesetzgeber fordert.
Wir dokumentieren für Sie.

